A quantity of dry air is slowly compressed in a cylinder by means of a piston, until its volume is halved. The initial pressure and volume are \(\require{color}{\color[rgb]{0.315209,0.728565,0.037706}1 \; bar \; \; (10^5\; Pa)}\) and \(\require{color}{\color[rgb]{0.079785,0.618358,0.483717}1 \;liter}\) respectively, and during the compression the pressure varies inversely with the volume. Sketch the process on a \(\require{color}{\color[rgb]{0.315209,0.728565,0.037706}p}-{\color[rgb]{0.079785,0.618358,0.483717}V}\) diagram and calculate the work transfer. Is this a positive or negative quantity?
Solution
Note that 1 liter is \(10^{-3} \; m^3\). Consider the diagram below; the work done is given by
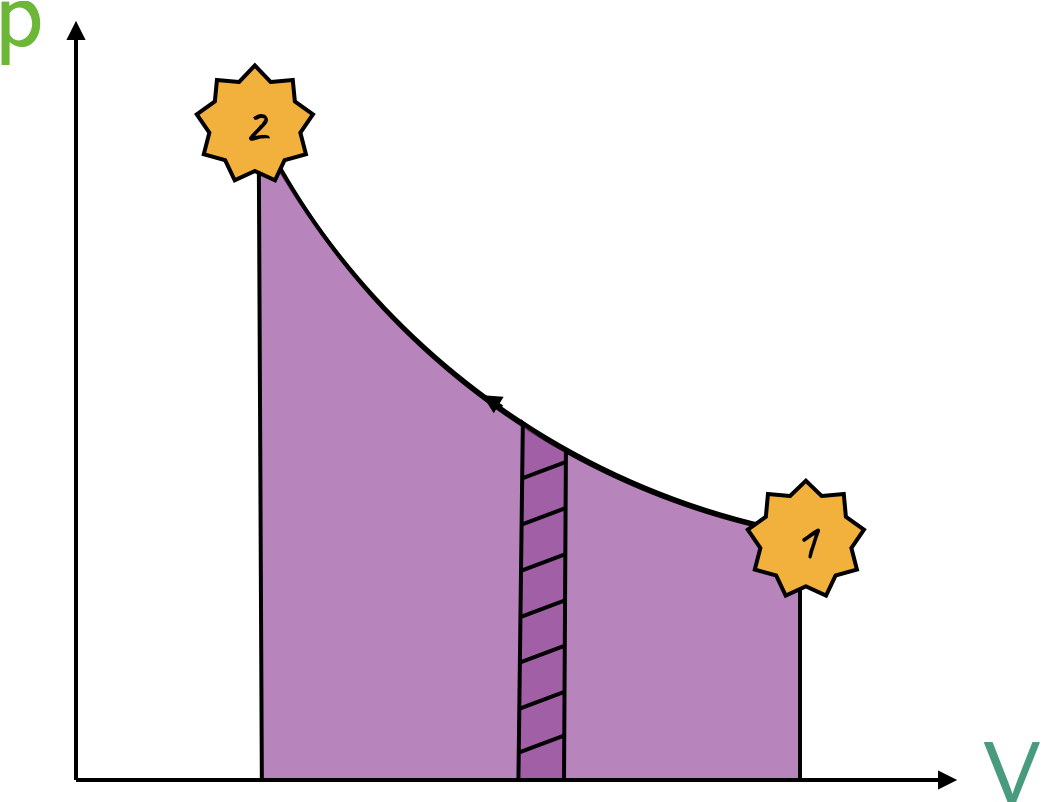
\[
\large
\require{color}
{\color[rgb]{0.562040,0.190215,0.568721}W} = \int_{{\color[rgb]{0.079785,0.618358,0.483717}V}_1}^{{\color[rgb]{0.079785,0.618358,0.483717}V}_2} {\color[rgb]{0.315209,0.728565,0.037706}p}d{\color[rgb]{0.079785,0.618358,0.483717}V} = \int_{{\color[rgb]{0.079785,0.618358,0.483717}V}_1}^{{\color[rgb]{0.079785,0.618358,0.483717}V}_2} \frac{C}{{\color[rgb]{0.079785,0.618358,0.483717}V}} d{\color[rgb]{0.079785,0.618358,0.483717}V} = \left[ C \; ln \left( {\color[rgb]{0.079785,0.618358,0.483717}V} \right) \right]_{{\color[rgb]{0.079785,0.618358,0.483717}V}_1}^{{\color[rgb]{0.079785,0.618358,0.483717}V}_2}.
\]
From the conditions at state 1, we have
\[
\large
\require{color}
C = {\color[rgb]{0.315209,0.728565,0.037706}p}_1 {\color[rgb]{0.079785,0.618358,0.483717}V}_1 = {\color[rgb]{0.315209,0.728565,0.037706}p}_2 {\color[rgb]{0.079785,0.618358,0.483717}V}_2,
\]
yielding
\[
\large
\require{color}
{\color[rgb]{0.562040,0.190215,0.568721}W} = {\color[rgb]{0.315209,0.728565,0.037706}p}_1 {\color[rgb]{0.079785,0.618358,0.483717}V}_1 ln \left( \frac{{\color[rgb]{0.079785,0.618358,0.483717}V}_2}{{\color[rgb]{0.079785,0.618358,0.483717}V}_1} \right) = {\color[rgb]{0.315209,0.728565,0.037706}10^{5} }\times {\color[rgb]{0.079785,0.618358,0.483717}10^{-3}} \times ln \left( \frac{{\color[rgb]{0.079785,0.618358,0.483717}1}}{{\color[rgb]{0.079785,0.618358,0.483717}2}} \right)
\]
Solving the above
