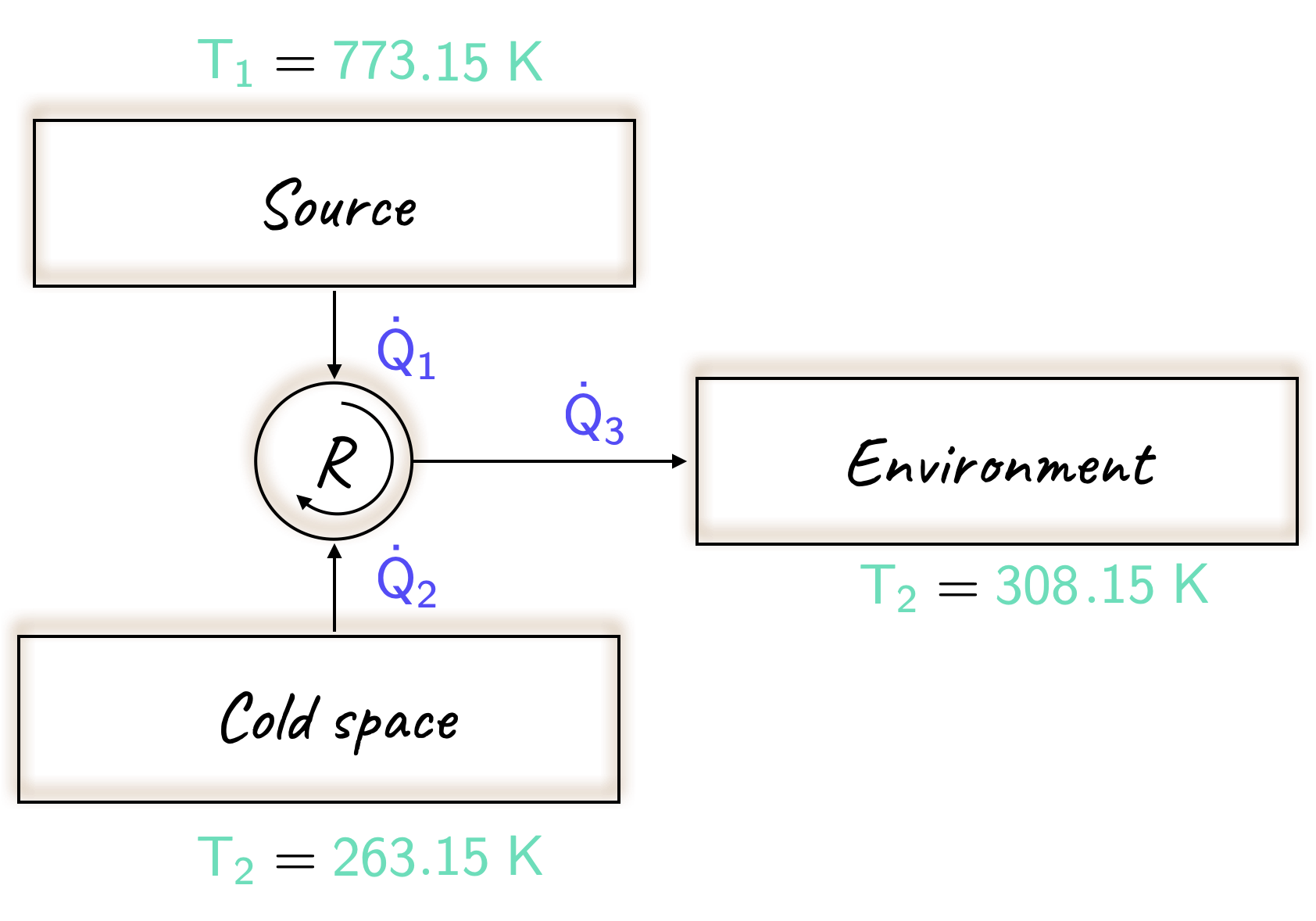
Solution
As before, it will be useful to sketch out a diagram of the process above.
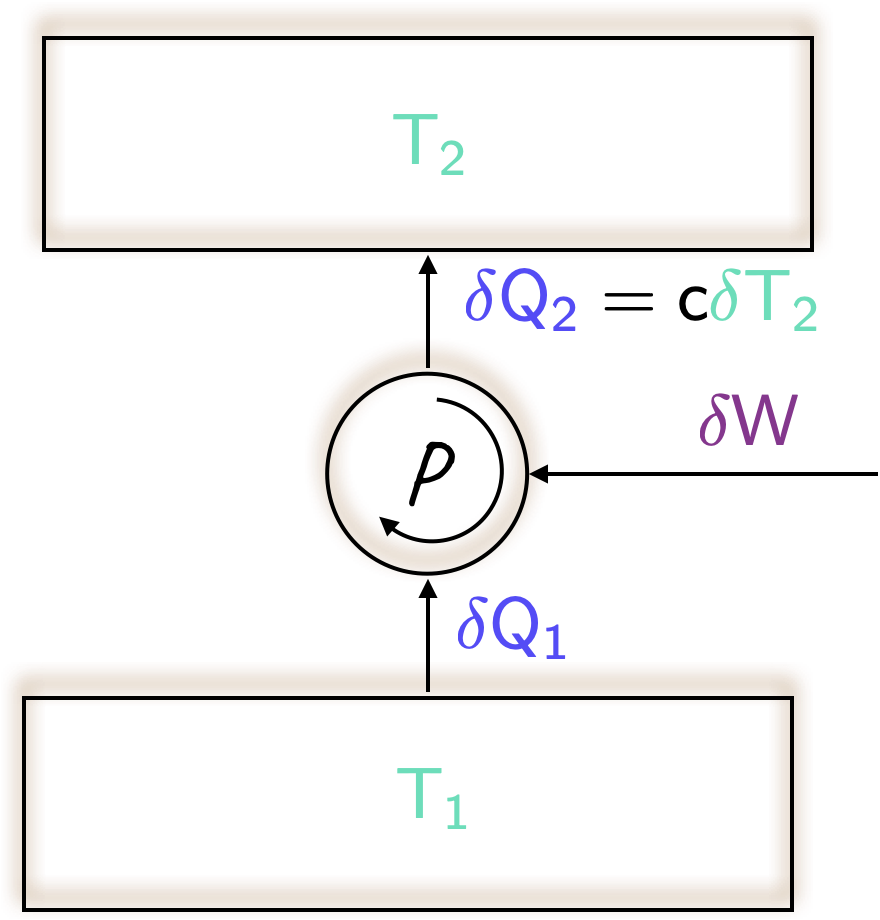
- From the first law, it is clear that
\[
\large
\require{color}
{\color[rgb]{0.334690,0.296180,0.998454}\delta Q_2}= {\color[rgb]{0.334690,0.296180,0.998454}\delta Q_1} + {\color[rgb]{0.562040,0.190215,0.568721}\delta W}.
\]
Note that if the pump were reversible, then it would be as efficient as possible, implying that the amount of work \(\require{color}{\color[rgb]{0.562040,0.190215,0.568721}\delta W}\) required by the pump would be as low as possible. This sets a lower limit on work, which consequently also sets a lower limit on \(\require{color}{\color[rgb]{0.334690,0.296180,0.998454}\delta Q_2}\), and therefore on \(\require{color}{\color[rgb]{0.164799,0.878862,0.723179}T_2}\).
- Consider the Clausius statement (with appropriate signs)
\[
\large
\require{color}
\frac{{\color[rgb]{0.334690,0.296180,0.998454}\delta Q_1}}{{\color[rgb]{0.164799,0.878862,0.723179}T_1}} - \frac{{\color[rgb]{0.334690,0.296180,0.998454}\delta Q_2}}{{\color[rgb]{0.164799,0.878862,0.723179}T_2}} \leq 0
\]
From the problem statement, we can infer that the constant \({\color[rgb]{0.000000,0.000000,0.000000}c = 1 \; MJ/K}\), leading to
\[
\large
\require{color}
\frac{c {\color[rgb]{0.164799,0.878862,0.723179}\delta T_2}}{{\color[rgb]{0.164799,0.878862,0.723179}T_2}} \geq \frac{{\color[rgb]{0.334690,0.296180,0.998454}\delta Q_1}}{{\color[rgb]{0.164799,0.878862,0.723179}T_1}}.
\]
Integrating both sides of this equation
\[
\large
\require{color}
\int_{{\color[rgb]{0.164799,0.878862,0.723179}300}}^{{\color[rgb]{0.164799,0.878862,0.723179}T_f}}\frac{c {\color[rgb]{0.164799,0.878862,0.723179}\delta T_2}}{{\color[rgb]{0.164799,0.878862,0.723179}T_2}} \geq \frac{{\color[rgb]{0.334690,0.296180,0.998454}Q_1}}{{\color[rgb]{0.164799,0.878862,0.723179}T_1}}
\]
\[
\large
\require{color}
\Rightarrow 1 \times ln \left( \frac{{\color[rgb]{0.164799,0.878862,0.723179}T_f}}{{\color[rgb]{0.164799,0.878862,0.723179}300.15}} \right) \geq \frac{{\color[rgb]{0.334690,0.296180,0.998454}600}}{{\color[rgb]{0.164799,0.878862,0.723179}300.15}}\Rightarrow {\color[rgb]{0.164799,0.878862,0.723179}T_f} \geq {\color[rgb]{0.164799,0.878862,0.723179}2217.82 \; K}
\]
Note that for the reversible case
\[
\large
\require{color}
{\color[rgb]{0.334690,0.296180,0.998454}Q_2} = \left({\color[rgb]{0.164799,0.878862,0.723179}2217.82} - {\color[rgb]{0.164799,0.878862,0.723179}300.15} \right) / c = {\color[rgb]{0.334690,0.296180,0.998454}1917.67} \; {\color[rgb]{0.334690,0.296180,0.998454}MJ}
\]
Thus the work input is
\[
\large
\require{color}
{\color[rgb]{0.562040,0.190215,0.568721}W} = {\color[rgb]{0.334690,0.296180,0.998454}1917.67} - {\color[rgb]{0.334690,0.296180,0.998454}600} = {\color[rgb]{0.562040,0.190215,0.568721}1316.7 \; MJ}
\].